Anion Gap
Anion Gap
We can calculate the anion gap from an ABG. This is the value of measured negative anions subtracted from the value of measured positive cations in plasma.
It is very useful in helping to work out the cause of a metabolic acidosis.
As HCO3– is the main buffering ion in the body, the anion gap indicates whether the pathology is caused by a primary disturbance of bicarbonate, or other anions.
Anion gap = [Na] + [K] – ([HCO3] + [Cl])
Normal range is 4 – 12 mmol/L
High anion gap
A high anion gap is almost always caused by an elevated concentration of anions.
An increase in anions causes an increase in H+ in the blood
HCO3– is used up to buffer the extra H+ ions and form H2O.
This causes a decrease in HCO3–, and so the anion gap increases.
Causes
Raised lactate – lactic acidosis (e.g., shock, ischaemia)
Raised ketones – diabetic ketoacidosis, alcoholic ketoacidosis
Raised urate – renal failure
High acidic toxins – salicylate/methanol poisoning
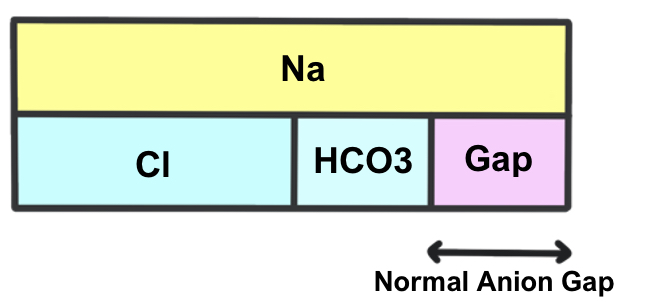
Normal anion gap
In a normal anion gap metabolic acidosis, there is a primary decrease in HCO3– ions.
This leads to a compensatory increase in Cl– ions to buffer the negative charge – so the anion gap stays constant.
Causes
HCO3– loss in GI tract – diarrhoea, intestinal fistula
HCO3– in renal tract – renal tubular acidosis
Chloride excess, e.g., ammonium chloride ingestion
Low anion gap
This is due to hypoalbuminemia. Albumin is the main unmeasured anion, so low levels cause a compensatory rise in HCO3 – and Cl– ions, decreasing the anion gap.
Disclaimer




